

The targeting domain is derived from a monoclonal antibody and recognizes a specific antigen. A single-chain variable fragment is generated by linking the variable region heavy and light chains. This domain allows the T cell to bind to the antigens and does not rely on dendritic cell antigen processing and presentation. Binding of the targeting domain to the target antigen triggers T cell activation.13,18
XA variety of antigen types are being investigated in B cell hematologic tumors. B cell malignancies express several potential antigen targets:
Connects the extracellular targeting element to the transmembrane domain and affects CAR function and scFv flexibility.19,20
These domains provide the co-stimulatory signal required for full T cell activation. Some evidence suggests that co-stimulatory domains may increase CAR T cell cytokine production and facilitate T cell replication. Co-stimulatory domains have also been shown to potentially reduce CAR T cell exhaustion, increase T cell antitumor activity, and enhance survival of CAR T cells in patients.10
X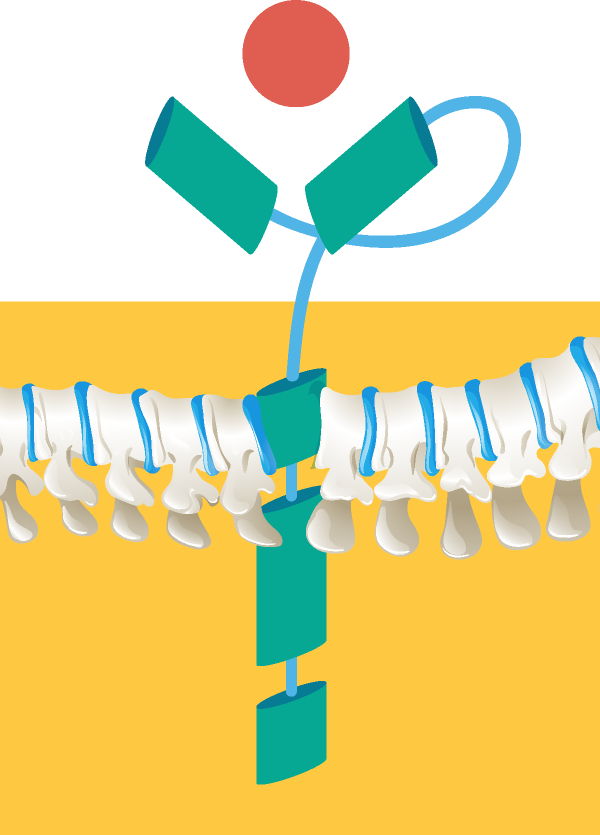

JANUARY 30, 2018. GREG GUTHRIE, ASCO STAFF
At the start of every year, the American Society of Clinical Oncology (ASCO) releases its Clinical Cancer Advances report. This in-depth report is published in ASCO’s Journal of Clinical Oncology and covers the major advances made in cancer care over the past year, including prevention, treatment, patient care, and a look toward the future. Each year, leaders in the oncology field also select a single area of research that achieved the greatest progress to name the Advance of the Year. In 2017, the FDA approved two CAR-T cell therapies for the treatment of acute lymphoblastic leukemia (ALL) in children and adult advanced lymphomas.
With CAR T-Cell Immunotherapy now available in Asia, Auxi Therapeutics Sdn Bhd is making the cancer treatment at very affordable and accessible to patients.
CAR-T cell therapy is a type of immunotherapy called “adoptive cell immunotherapy.” As ASCO President Bruce E. Johnson, MD, FASCO, describes it, this technique “allows clinicians to genetically reprogram patients’ own immune cells to find and attack cancer cells throughout the body.”
In CAR-T cell therapy, a person’s T cells are removed and taken to a laboratory. The T cells are genetically changed so they will attack cancer cells. These CAR-T cells are grown in large numbers and then infused back to the patient. One of the remarkable things about this treatment is that it is a “living therapy.” CAR-T cells typically have to be infused only once, because they go on to multiply in the body. CAR-T cells continue fighting the cancer in the patient’s body, and their efectiveness may even grow over time.
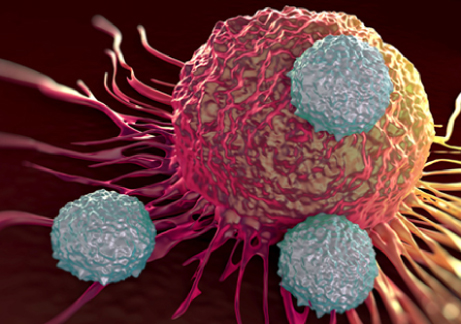
The figure showed scanning electron microscope image of 3 CAR-T cells (in grey) attacking a leukemia cell (in red). The binding of the CAR-T cell to the tumour cell initiates an immune defence against the cancer cell