

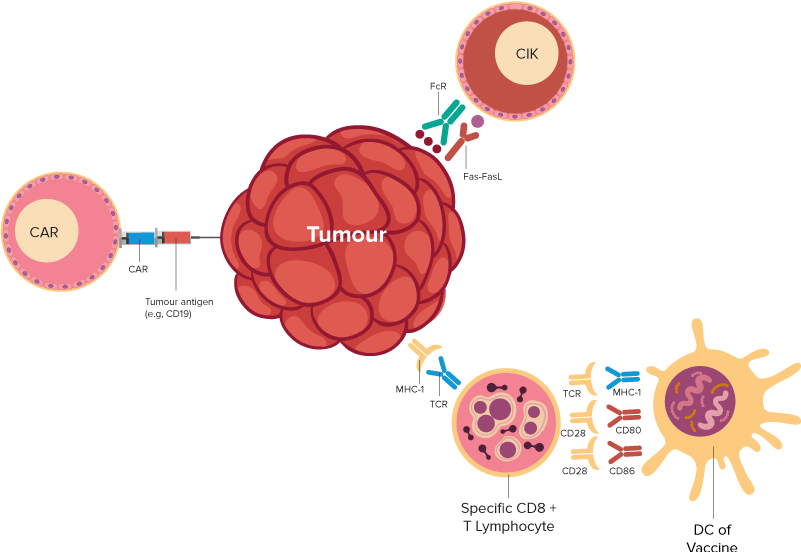
T cells are immune cells that fight infection. In T-cell immunotherapy, some T cells are removed from a patient’s blood. Then, the cells are changed in a laboratory, so they have specific proteins called receptors. The receptors allow those T cells to recognize the cancer cells. The changed T cells are grown in the laboratory and returned to the patient’s body. Once there, they seek out and destroy cancer cells.
| T-Cell-based Immunotherapies | Indication | References |
|---|---|---|
| Autologous/Allogenic CAR-T (Chimeric Antigen Receptor) Cell Therapy - AuxiCART Different of Targeted Antigen, as follows, | Hematology Malignancies:
| 1-17 |
| Autologous Immune Cell Therapy, DC-CIK (Dendritic cells – Cytokine-Induced Killer): 3 types of therapies, as follows, Auxidendtrix (DC Immunotherapy) Xiltokine (CIK Immunotherapy) Audenkine (DC-CIK Immunotherapy) | Hematology Malignancies:
Solid Cancers:
| 18-30 |
This decade, a lot of clinical trials regarding CAR-T therapy were carried out around the word. As of April 19, 2018, the information from Clinicaltrails.gov showed that more than 270 CAR-T related studies were undergoing clinical trials in many different countries.
By analyzing the overall response rate (ORR) and complete response rate (CRR) of CAR-T therapy in patients with different tumors, we found that the response rate was significantly higher for patients with hematologic malignancies compared to patients with solid malignancies.